Operational Performance and Optimization of RO Wastewater Treatment Plants
Craig Bartels, Rich Franks, and Keith Andes
Hydranautics, 401 Jones Rd. Oceanside, CA 92058 (E-mail: cbartels@hydranautics.com)
There has been a rapid growth in the use of RO membranes for wastewater reclamation. The membrane process offers high removal rates for many contaminants and pollutants, as well as operating at low energy consumption. It is very important that these commercial wastewater treatment plants operate consistently to minimize down-time and maximize membrane life. Much has been learned about the optimum design and operation of a WWRO system from the many small to mid-size WWRO plants that have been installed since the late 1970’s. More recently, there have been a number of mega-sized WWRO plants have come about due to the confidence in designing and running the smaller plants.
Flux rate, recovery and membrane element type continue to be evaluated. Most WWRO plants today use flux rates in the 17-20 lmh range and recoveries in the 75-85% range. Further trials and studies are under investigation to determine if these ranges can be extended. Some work with 16 inch membranes and special treatment conditions suggest that more aggressive flux rates may be possible. Also, interstage pressure boost designs and new membrane elements with thicker, low fouling spacers are able to provide better flux balance in the system. The energy consumption of these new alternatives will be considered. There is also much data now available to characterize the potential contaminant removal efficiencies possible with advanced membrane technology. These are able to produce product water with the required purity. Rejection of different ions and organic contaminants is reported.
There is an increasing use of reverse osmosis (RO) in the reclamation of wastewater. Relative to other technologies, the main drivers for this include the low energy consumption of RO and the high rate of contaminant removal. Also, the reliability of these plants has greatly improved, giving developers confidence in the supply of water from this technology. These factors have been a key to the acceptance of this technology.
RO treatment of wastewater started back in the late 1970’s with small plants, such as the one at Orange County Water District. The experience gained from the many years of operation of existing plants has been an essential aspect to the growth of this technology. This is especially evident in the fact that many mega-sized wastewater RO plants have been built in the last five years (Figure 1). Included in these plants are the 320,000 m3/d plant in Sulaibiya, Kuwait, the 280,000 m3/d Orange County Ground Water Replenishment (GWR) plant in the USA, and the 228,000 m3/d plant in Changi, Singapore. These have all become key installations to support the water-short communities where they are located.
| FIGURE 1 Historical Development of RO for Wastewater Reclamation |
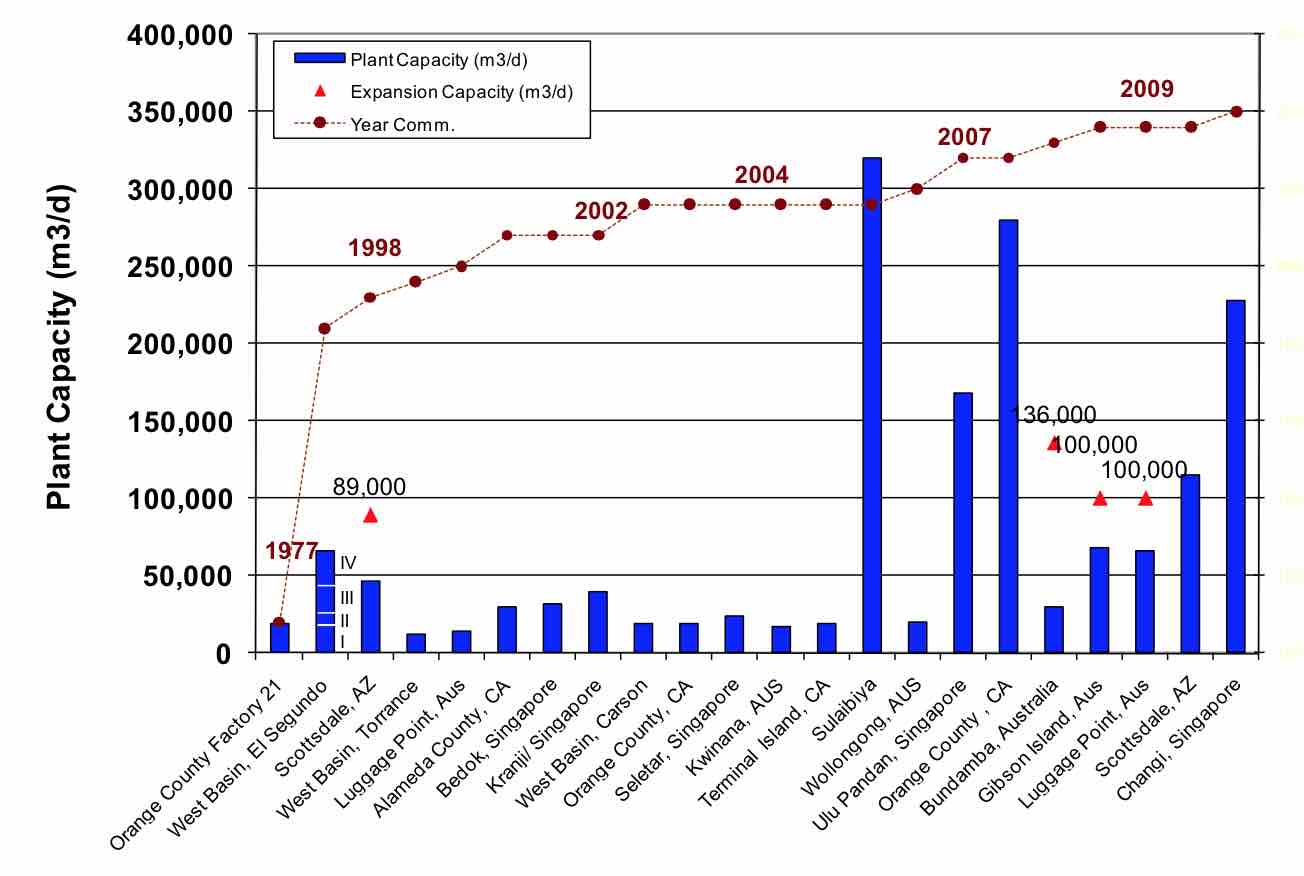 |
The operation of these mega-plants has focused on lower energy of operation, consistent on-line time, and extended membrane life. The most common process used for wastewater reclamation is the use of conventional activated sludge process, followed by UF or MF membrane filtration, RO desalination, and UV disinfection. (Seah 2003, Daugherty 2005) More recently, there has been a move to replace the conventional activated sludge and membrane filtration processes by a membrane bioreactor. (Tao 2008) In either case, as long as there is effective filtration of colloidal material from the in-coming feedwater, and there is chlorine dosing to maintain a few parts per million (ppm) of chloramines, the RO membranes can operate very stably. (Bartels 2003). Beyond this basic and successful approach, there have been a number of new process improvements.
There are many process features that are options for consideration when designing the wastewater plant using RO reclamation. The engineers must choose the type of membranes, membrane array configuration, one or two-pass membrane scheme, use of interstage boost pumps, flux rates, recoveries, scale control chemicals, cleaning strategies, and biological control methods. One of the key features is the fouling rate of a given membrane on a given wastewater feed. There have been reports of significant permeability loss due to fouling of the RO. Wastewater contains many contaminants which can foul the RO membrane. Due to the variability of the wastewater, this can greatly affect the expected energy consumption for treatment. In one case (Walker 2007), there were reports that certain industrial chemicals we causing the membranes to lose 40% of their permeability. Also, there have been reports of the differences in fouling due to the type of membrane. (Alexander 2003) Thus, it is important to understand these characteristics before selecting the membrane. These issues will be reviewed in more detail later in this paper.
Another critical feature about the operation of RO wastewater plants is the removal of contaminants from the wastewater. The quality of the filtered water is critical for the type of use anticipated. In one regard, industrial reuse of reclaimed wastewater is most restrictive. Treatment plants like those at West Basin can use the reclaimed water for low and high pressure boilers, process water or irrigation. (Abi-Samra 2002) In places like Singapore, the water is primarily used by the semi-conductor industry for wafer fabrication. In all of these applications, the reclaimed water must conform to very strict purity requirements. One example of the water quality requirement for West Basin is given in Table 1.
Table 1 Typical Water Quality Targets for Industrial Reuse
| Limit: Instantaneous | Limit: Rolling Average | |
| (mg/l) | (mg/l) | |
| Calcium | 2 | 1 |
| Magnesium | 1 | 2 |
| Amomonia | 5 | 4 |
| Silica | 2 | 1 |
| Dissolved Solids | 50 | 35 |
Achievement of this performance is totally dependent on the type of membrane selected. High rejection membranes can achieve these targets, but there is a trade-off with energy consumption. Generally, the higher the rejection of the membrane, the more pressure it requires. In some cases, the water quality is so strict, such as with high pressure boiler feedwater, the permeate of the first pass must be treated a with a second pass RO system. Therefore, it is critical to understand the rejection characteristics of each membrane type and assure that it can meet the required product water quality.
More recently, there has been increasing use of RO in reclamation of wastewater for indirect potable use. For example, the recent implementation of the GWR facility in Orange County produces 280,000 m3/d of treated wastewater that is used to augment the groundwater in the region that supplies local municipalities with drinking water. (Franks 2004) RO plays an integral role in the advanced treatment process used at this plant. At this plant, low pressure, high rejection ESPA2 membranes are used to make RO permeate with less than 50 mg/l TDS. But more importantly, RO membranes are extremely valuable for ensuring that the reclaimed water is safe for potential potable reuse. Wastewaters can contain a range of organic contaminants, including pharmaceutical compounds, personal care products, pathogens, disinfection by-products, and pesticides. Due to their complex structure, they are often poorly degraded by bacteria during the activated sludge process. Also, due to their water solubility, they can stay dissolved in water and not be removed in the sludge. Thus, they can present a threat to the safety of reclaimed water, making RO separation a key step in the safe recovery of water from wastewater sources.
In one study (Snyder 2008), a survey of plant effluent quality from a drinking water plant and an Advanced Wastewater Treatment plant was compared. The data shows that Atrazine, a pesticide, was found at levels of 76-1080 ng/L for the potable water plant, while they were less than 0.25 ng/L from the RO water reuse plant. This indicates the problem with these type of contaminants, they are not readily removed by the convention technology used in potable water treatment plants, and it shows that Advanced Wastewater Treatment plants can give the added level of protection through the use of RO.
As previously mentioned, the typical advanced wastewater treatment process consists of grit removal, primary treatment, conventional activated sludge treatment, clarification, micro or ultra-filtration, chemical addition, RO treatment followed by UV disinfection and product water stabilization. (Figure 2.)
| Figure 2 Advanced Wastewater Treatment process steps. | |
 |
 |
| a. Grit Removal | b. Primary Treatment |
 |
 |
| c. Activated Sludge Treatment | d. Clarification |
 |
 |
|
e. UF/MF Filtration
|
f. RO Purification |

|
 |
|
g. UV Disinfection
|
h. Recarbonation |
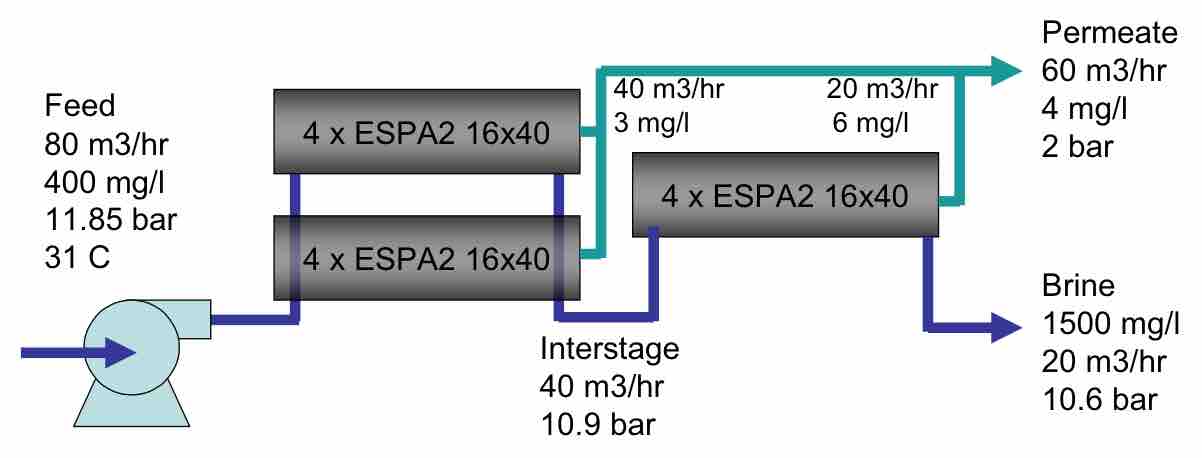
A typical process design, such as that at the original Bedok plant, is shown in Figure 3. The RO is designed with 4 trains operating at 75% recovery with 2 stages, 7 elements per pressure vessel and a 17.6 lmh flux. A few of the key choices in any design are the recovery and the flux rate.
| FIGURE 3 Bedok Wastewater treatment process |
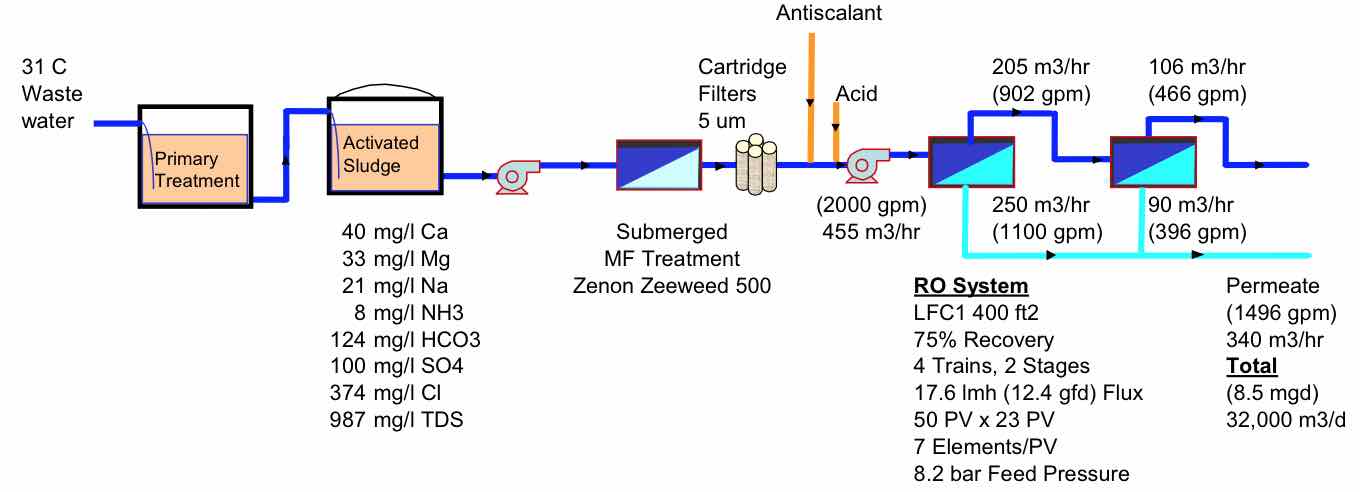 |
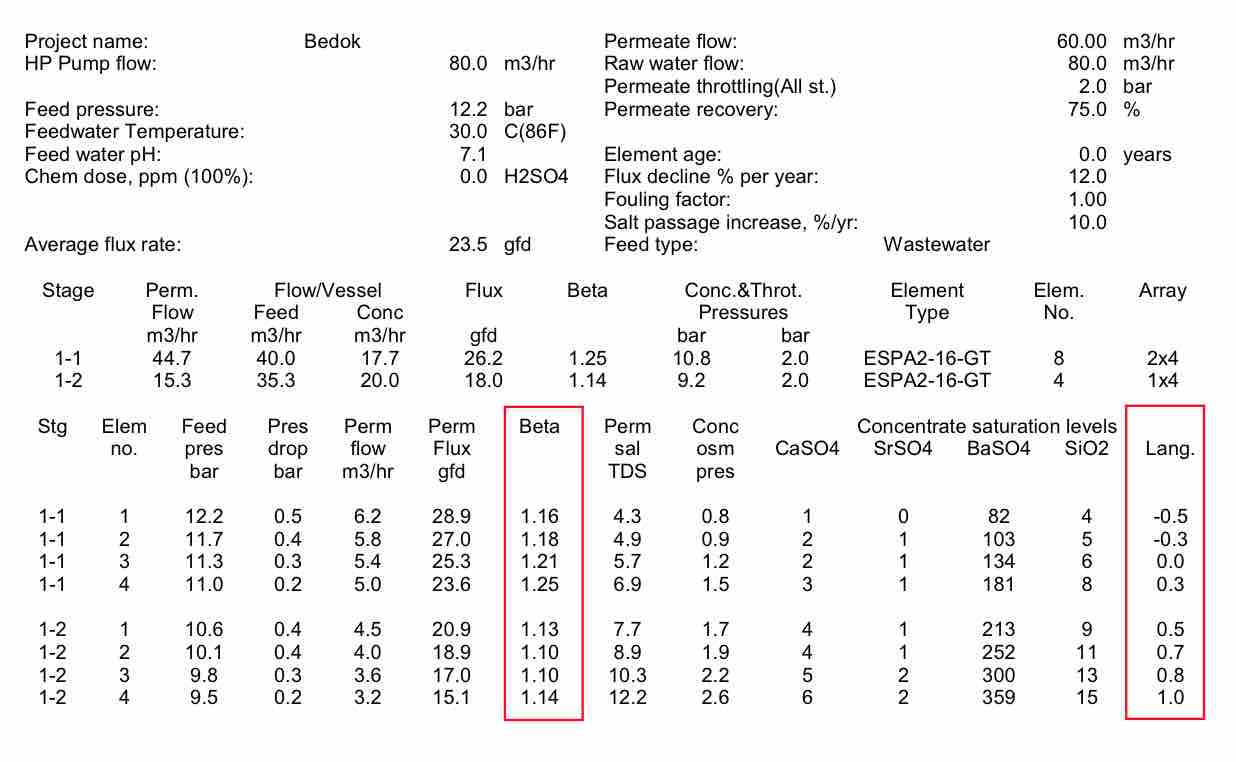
In most RO wastewater treatment plants, the flux rate for the RO process is chosen in the range of 17 – 20 lmh. This is based on extensive research at pilot tests and commercial plants. Flux rate is important in a RO system because it governs the crossflow rate and mass transport rate through the membrane. The higher the mass transport rate, the more solids are drawn to the membrane surface. Spiral wound RO systems are based on the concept that the crossflow sweeps solids from the surface and keeps the membrane from becoming significantly fouled. There is an optimum ratio of crossflow to permeate flow to keep the membrane surface clean, and the energy consumption at a minimum. Also, if the crossflow becomes too low, the concentration polarization at the membrane surface becomes high. This causes the apparent ion and contaminant concentration at the surface of the membrane to be much higher than the incoming feed. Beta value is the measure that is used to determine the degree of concentration polarization. A beta value of 1.2, or 20% higher concentration at the membrane surface, is considered to be the maximum beta value that is typically allowed.
Using the system at West Basin as an example, Table 2 shows the flux, flow and beta values by element in the train. It can be seen that the beta values are lower in the front of the first stage, and higher toward the rear of the first stage. Even though the lead elements have the highest permeate flux, they have a low beta value because of the high crossflow at the front of the vessel. The higher beta values at the end of the stage can lead to slightly higher salt passage and higher pressures. The bigger concern with beta is in the second stage. It can be seen that the beta values are around 1.06, because the low permeate flow caused by the high system osmotic pressure. As can be seen, the LSI, or carbonate scaling tendency, becomes much higher in the tail elements of stage 2. This makes the likelihood of scaling more prevalent. High beta values at the end of stage 2 would increase the chance of scale formation.
| Table 2 Projected individual element performance for the West Basin RO System |
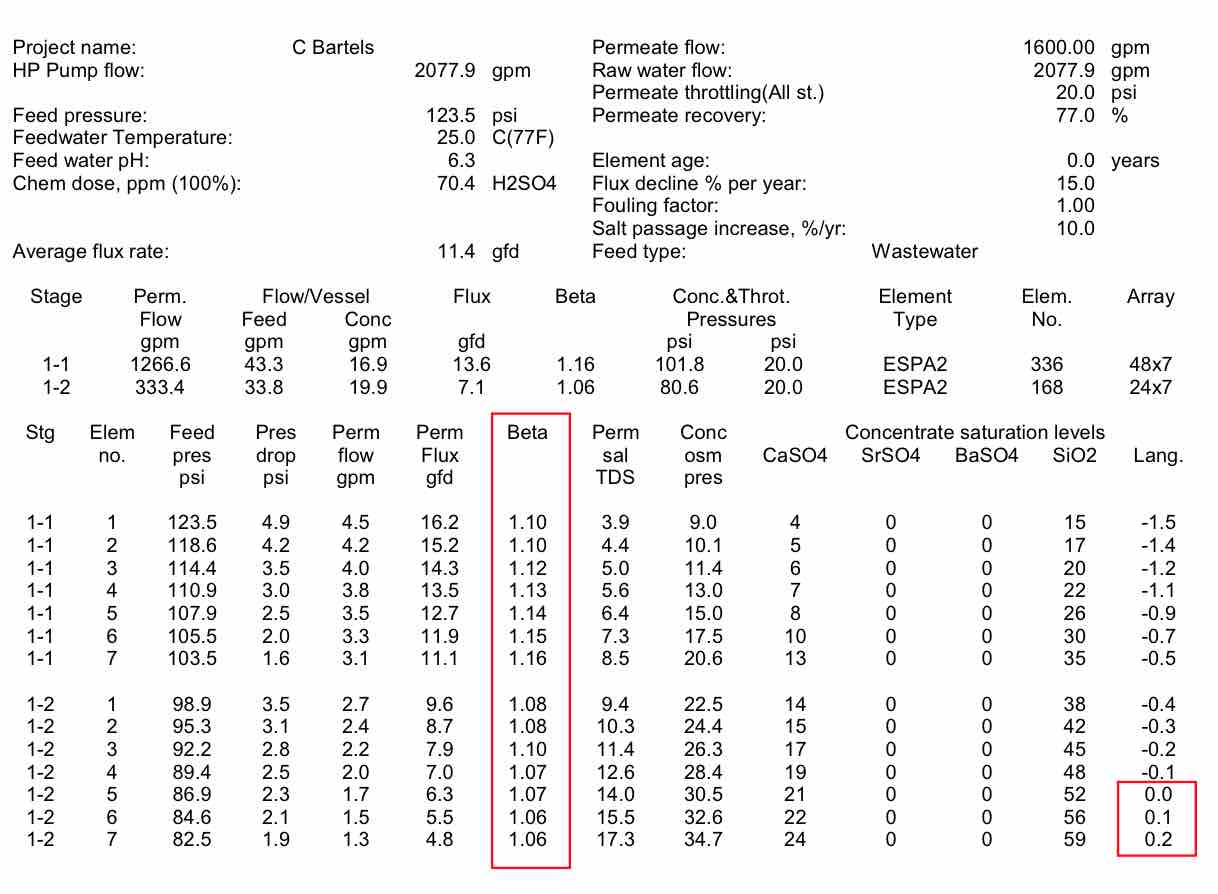 |
It can be seen that the flux rate at the tail of the system can be rather low, due to the rising osmotic pressure. This is common in most WWRO plants. In some cases, flux can go to almost zero at the last element. An example of this is shown in the design options shown in Table 3. One alternative is to raise the flux in the second stage through the use of an interstage boost pump or energy recovery turbine (Table 3), or the use of elements with thicker spacers that lower differential pressure losses (Table 3). It can be shown that the latter case gave the lowest energy consumption (lowest pressure), except for the high capital cost case where a turbo was included.
|
Table 3 Comparison of Various WWRO Designs for 30 C Feed Temperature, 1200 mg/l Feed TDS and 20 lmh Flux. |
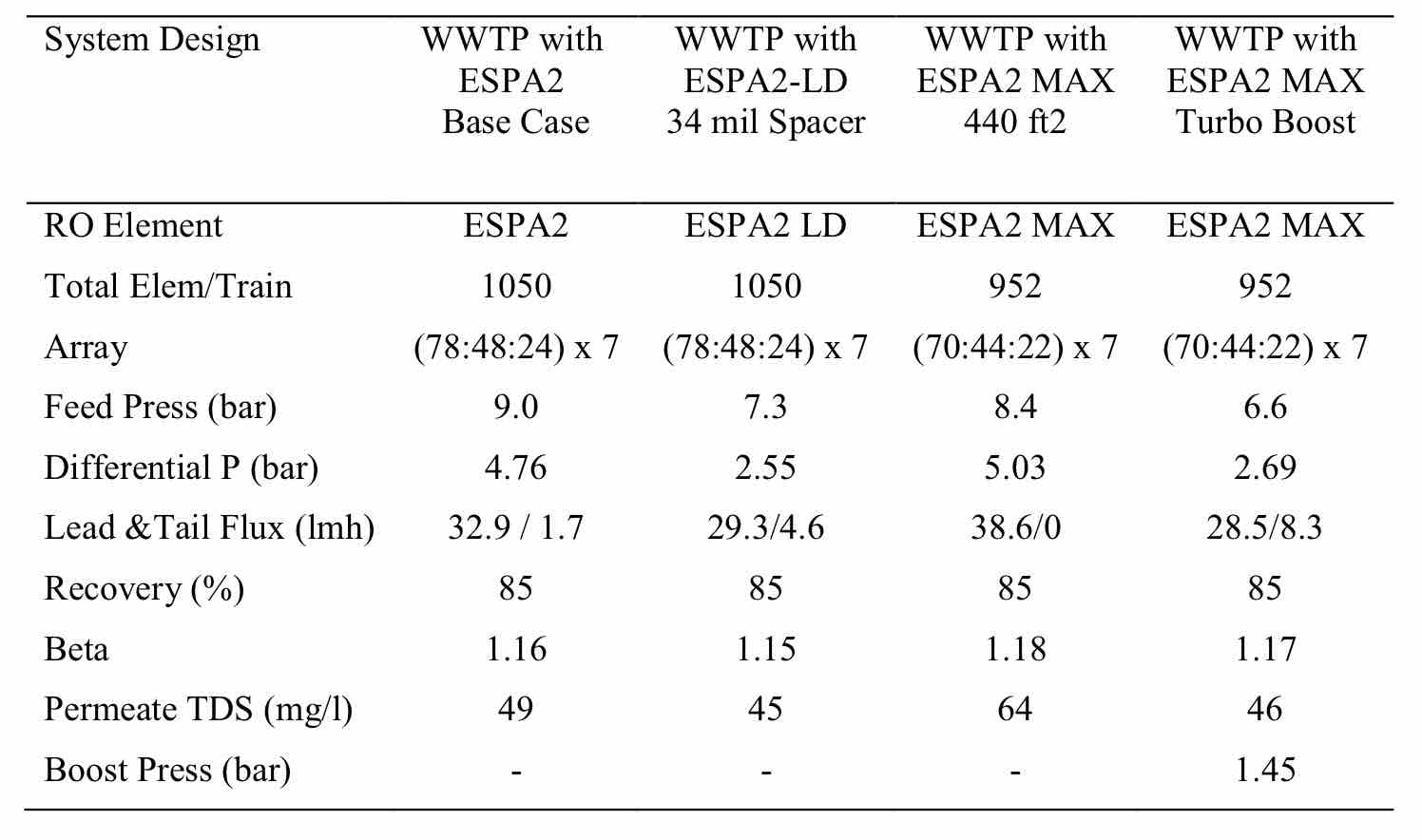 |
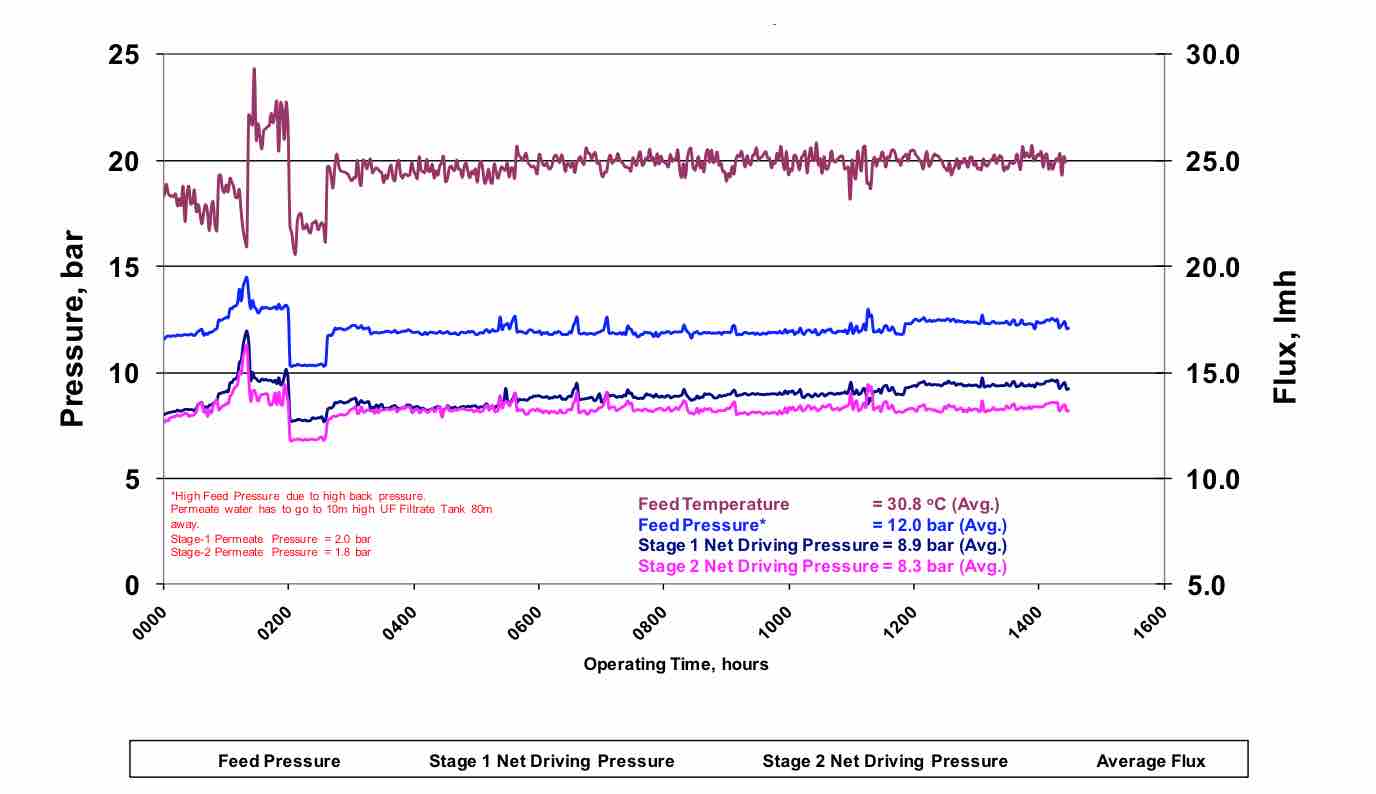
Figure 4 shows the operational result for a plant that utilized a flux rate of 18.6 lmh, maintained all critical system parameters and utilized 80% recovery. As with any RO wastewater plant, there is an initial permeability decline due to adsorption of organic contaminants, followed by a steady state operation with minimal permeability decline.
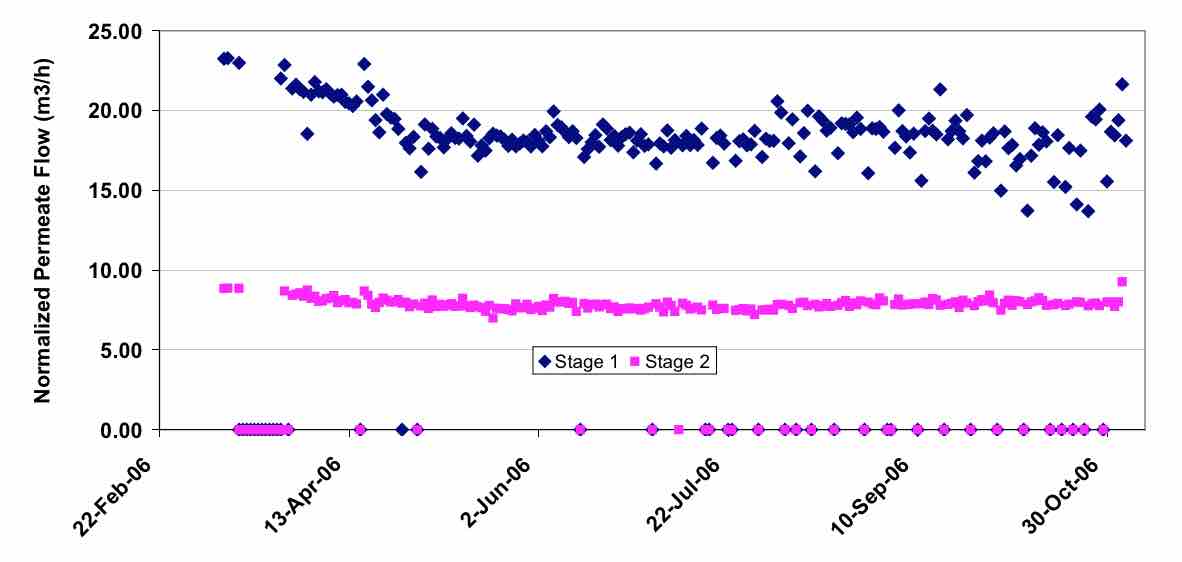
In contrast, some plants operate at more aggressive average flux rates. In one study on a municipal wastewater with UF pretreatment (Franks 2004), the flux rate was increased from 15.3 lmh (9 gfd) to 22 lmh (13 gfd), and there was clear evidence the fouling rates were increasing at the higher flux rate, as evidenced by the declining normalized flow. (Figure 5) This is believed to be due to insufficient crossflow to keep the membrane surface clean. Such increased fouling can lead to higher cleaning costs, more plant down time and higher energy costs. Thus, it is important to balance the process design to maintain stable operation with high flux, lower capital cost.
More recently, there has been a demonstration of new technology to control fouling at higher fluxes, which is being used on large diameter elements. GrahamTech has promoted the use of a novel flow distributor on the front end of an element, the use of EMF field from a coil embedded in the pressure vessel around the RO elements, and the use of daily permeate flush to maintain a flux rate nearly double the ordinary flux. (Ng 2007) A basic flow scheme for a pilot operated at the Bedok wastewater plant is shown in Figure 6.
|
Figure 4 Stable Operation of a RO wastewater system at 18.6 lmh flux and 80% recovery. |
 |
|
Figure 5 Effect of Flux on the Fouling Rate of WWRO Process |
 |
| Figure 6 Graham Tech high flux, 16 inch element process used at Bedok WWTP. |
 |
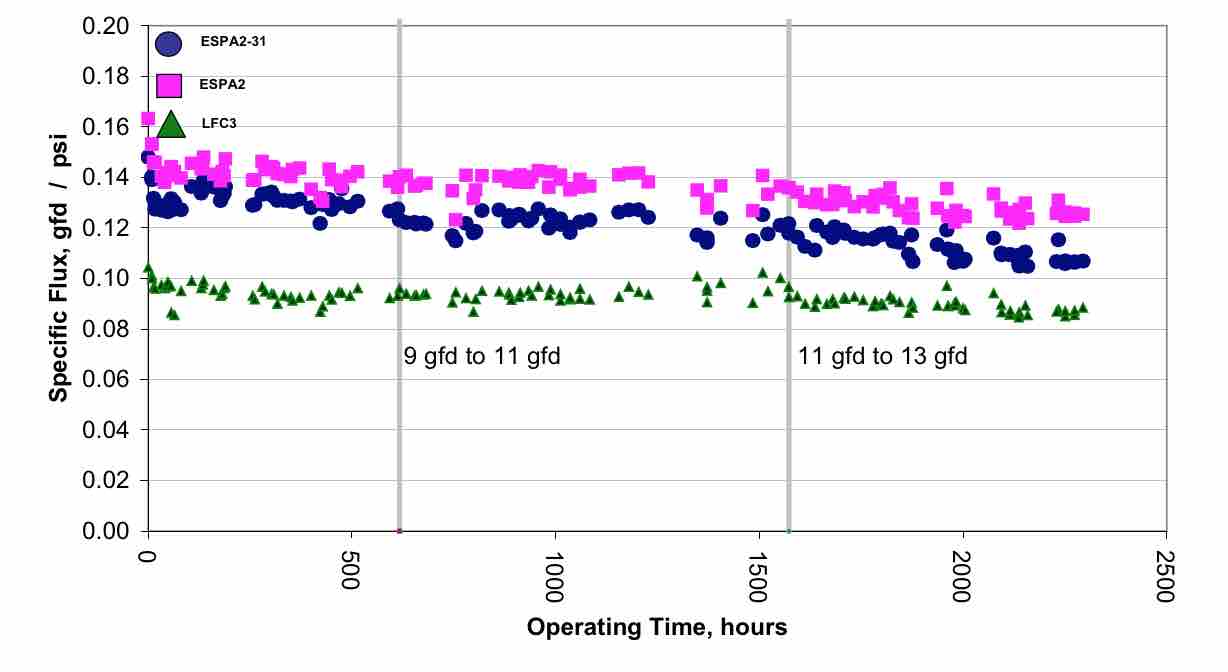
It can be seen that the flux rate is about twice that of the flux rate typically used in RO wastewater plants. They also used 4 elements per pressure vessel instead of the typical 6 or 7 elements per pressure vessel. When considering the concentration polarization, or beta value, the values are higher (Table 4) than seen in the previous example (Table 2). It can be seen that some elements have beta values higher than the usual value of 1.2. However, long term operation has shown that stable operation was achieved. (Figure 7) This result indicates that in some cases it may be possible to operate at higher flux rates. Higher flux will result in significant capital savings.
| Table 4 Projected individual element performance for the Bedok 16 inch RO Pilot |
 |
| Figure 7 Performance trend for 16 inch Bedok Pilot Unit |
 |
As previously mentioned, another key aspect of RO system design for wastewater reclamation is the removal of contaminants. There are a variety of water constituents that must be controlled and measured at a RO wastewater treatment plant. These typically include TDS, hardness, nitrates, nitrites, phosphates, silica, TOC, organic nitrogen, synthetic chemicals, pharmaceuticals, pesticides, and boron.
For industrial plants, the biggest concern generally is associated with ionic constituents that can form scale in low and high pressure boilers; these include hardness, silica and phosphates. Chart 1 shows the rejection of these compounds by RO membranes. It can be seen that high rejection membranes like ESPA2 and LFC1 have very high rejection of hardness ions. In most cases, rejection was better than 99.9%. This high rejection is due to the hardness ions having a divalent charge. Silica, with only a monovalent charge, is more difficult to remove with RO membranes. The chart show that the rejection can vary from 99.0 to 99.8%. In most cases, this is sufficient to meet the silica requirements listed in Table 1. Sulfate ion, being divalent and negative, is also well removed, with rejections in the range of 99.7 to 99.98%. The membranes also have high rejection of phosphate, which is also multivalent anion. Rejection of this ion is around 99.7 to 99.99%.
However, the high rejection of these ions can lead to scale formation in the RO system when operated at high recovery. Most RO wastewater processes operate at 75 to 85% recovery. For example, the Bedok and Kranji plants have been operated at 75% recovery (Bartels 2003), the Ulu Pandan pant in Singapore runs at 80% recovery and the Orange County GWR Plant runs at 85% recovery (Franks 2008). The recovery is limited by the concentration of the scale forming components in the feed water.
| Chart 1 Summary of Rejections for Various Constituents in Wastewater by RO Membranes |
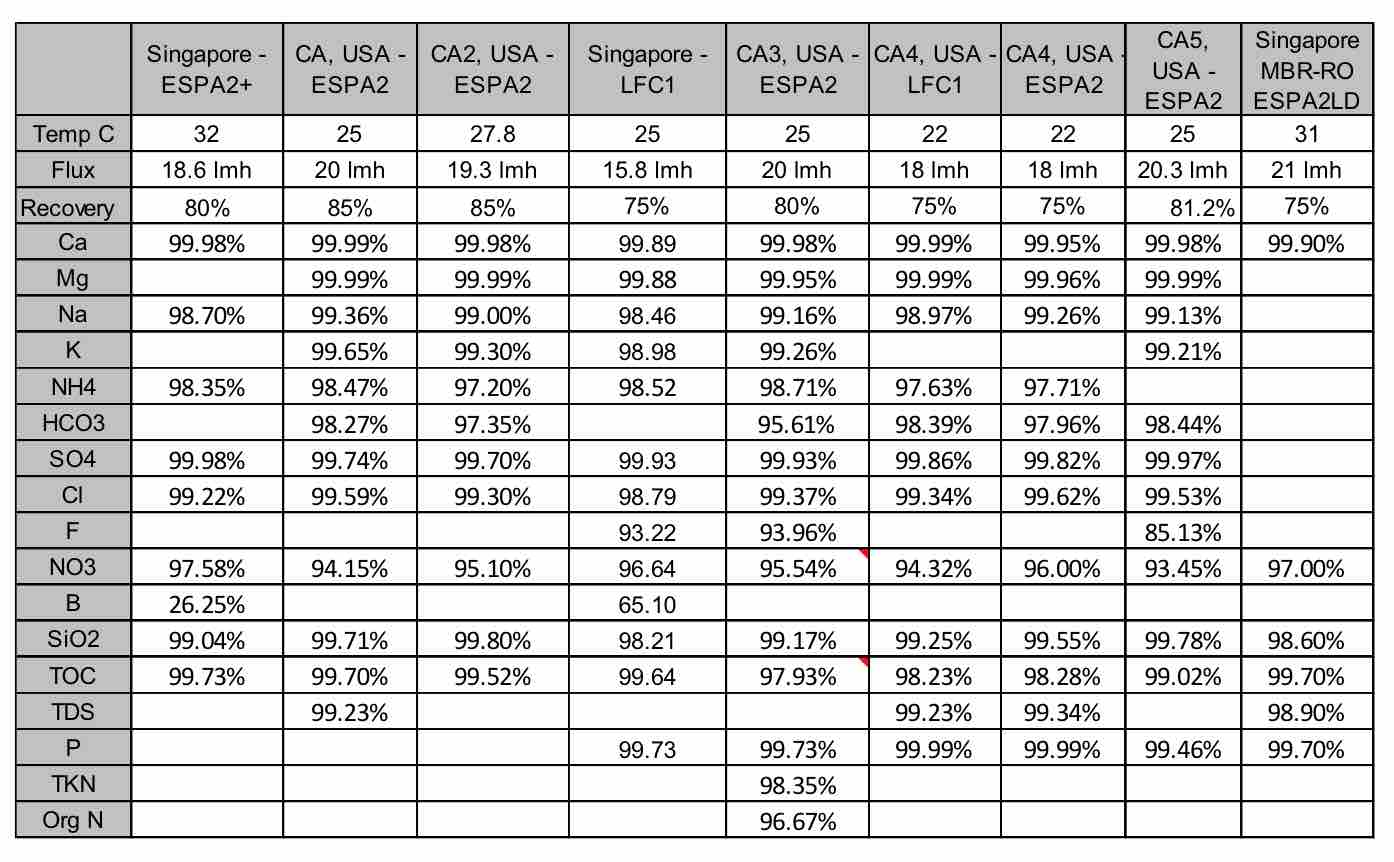 |
For other industrial plants, such as those in Singpore which provide water to wafer fabrication plants, TOC levels are critical and limited to 100 ppb, with a typical value of 50 ppb expected. Chart 1 shows the TOC rejection varies from 98.2 to 99.7%. Since TOC characteristics vary greatly, this can lead to variation in rejection. In most cases rejection of 99.7% is sufficient to reduce TOC well below the 100 ppb level.
In addition to industrial uses, there has been a growing use of RO wastewater treatment to augment potable water supplies by indirect use. For this application, other constituents are of concern. Organic contaminant removal is of particular concern, as well as nitrates. Although the rejection of TOC is quite high, some small organic molecules such as pharmaceuticals and carcinogenic compounds may not be as highly rejected. In many cases though, the rejection of RO membranes is sufficient to meet current requirements. However, further study on pharmaceutical compounds, hormones, and pesticides is needed. One report (Daughtery 2005) based on the GWR Phase 1 Plant at Orange County, indicates that the membranes produced permeate with halogenated compounds < 0.5 ug/l, PCB’s < 0.5 ug/l, NDMA < 5 ug/l, caffeine < 0.3 ug/l, and estrone < 10 ng/l. These results indicate that the membrane is very efficient in removing such difficult to treat compounds. Another study on these compounds was done with fresh membrane samples (Kimura 2003) and indicated that higher molecular weight species are highly rejected, as expected. However, rejection of lower molecular weight compounds is not highly rejected.
A new study is underway to characterize the rejection of these chemical types using membrane samples from actual wastewater plants. The organic fouling of membranes is common and gives rise to higher rejections. It is possible that this will sufficiently increase rejection and give satisfactory rejection. However, it may be possible that cleaning of the membrane may result in higher passage of such chemicals. This issue is also under investigation and will be reported.
Performance of operating plants has given much important information to help optimize the design and operation of commercial plants. Typical flux values are in the range of 17 – 20 lmh because of the extensive research which has shown that this is a sustainable flux. However, new information has shown that higher flux rates can be achievable with additional, new technology.
It is also important that there is some balance of flux in the system. Because of the high recoveries used in some cases, the flux rates of tail elements in the last stage can be very low. To compensate for this, lead elements must operate at higher fluxes. This can be compensated by using booster pumps or turbo boost pumps. More recently, new membranes have come on the market with have thicker spacers and are more fouling resistant. The use of these membranes reduces the pressure losses, helping to improve flux balance in the system by providing more net driving pressure in the last stage.
Finally, data was reported to show that state-of-the-art low pressure, high rejection membranes can achieve 99.95% or more rejection of hardness ions and TOC removal of more than 99.5%. Production of such a pure permeate is critical if the water is to be used for industrial purposes. New research is underway to characterize membrane rejection of synthetic chemical contaminants. These include pharmaceuticals, personal care products, carcinogens, and other pollutants. Initial data from some plants, such as the OCWD plant, suggest that rejection of these types of contaminants may be satisfactory for current regulation limits.
- Abi-Samra B.C., Cook P., Lange P., Zepeda J., (2002) “Design and operation of a microfiltration and reverse osmosis system treating secondary effluent and providing high quality reclaimed \water”, Proceedings of the AMTA Conference. Tampa, FL, USA.
- Alexander K., Alt S., Owens E., Patel M., McGovern L., (2003). Low fouling reverse osmosis membranes: Evidence to the contrary on microfiltered secondary effluent, Proceedings of the AWWA Membrane Technology Conference. Atlanta, GA, USA.
- Bartels C., Wilf M., Andes K., Iong J., (2003). Design considerations for wastewater treatment by reverse osmosis, Proceedings of the International Desalination and Water Reuse Conference, Tampa, FL, USA.
- Daugherty J., Alexander K., Cutler D., Patel M., Deshmukh S., (2005). Applying advanced membrane technology for Orange County’s water reuse treatment facilities, Proceedings of the AWWA Membrane Technology Conference. Phoenix AZ, USA.
- Franks R., Papukchiev U., and Murkute P., (2004). MF/RO membrane system for treatment of municipal secondary effluent wastewater”, Proceedings of the Annual Water Reuse Conference, Phoenix, AZ, USA.
- Franks R., Bartels C., Andes K., Patel M., and Young T. X., (2007) Implementing energy saving RO technology in large scale wastewater treatment plants, Proceedings of the International Desalination and Water Reuse Conference, Las Palmas, Spain.
- Kimura K., Amy G., Dreses J., Heberer T., Kim T. U., Watanabe Y., (2003) “Rejection of organic micropollutants (disinfection by-products, endocrine disrupting compounds, and pharamaceutically active compounds) by NF/RO”, Journal of Membrane Science, 227 113- 121.
- Ng, H.Y. , Tay, K G., Chua, S.C., Seah H., (2007) Performance of novel 16-inch (Super Flux) RO demonstration systems for water reclamation, Proceedings of the International Desalination and Water Reuse Conference, Las Palmas, Spain.
- Seah H., Poon J., Leslie G., Law I.B., (2003). Singapore’s NEWATER demonstration project another milestone in indirect potable reuse, Water, June.
- Snyder S., (2005) Private Communication.
- Tao G., Kekre K., Qin J., Oo M.H., Viswanath B., Lew C.H., Chong M.L., Kan L.M. Seah, H.(2008) MBR and RO integrated system for water reclamation and purification, Proceedings of the SIWW Conference. Singapore.
- Walker T., Stewart C., Brewster M., and Longfield P., (2007) Industrial waste organic fouling on membrane recycling plants on Australian experience, Proceedings of the Ozwater Conference, AUS.
