Basics of Membrane Technology
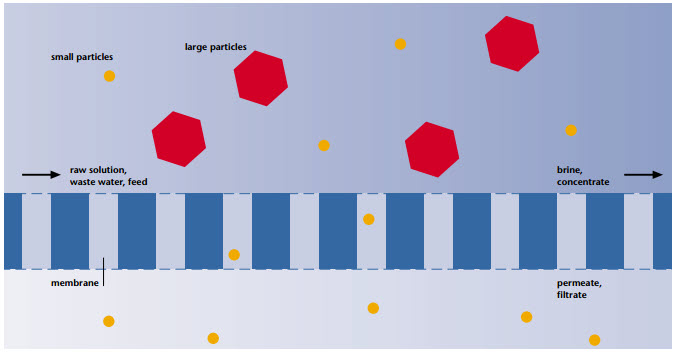
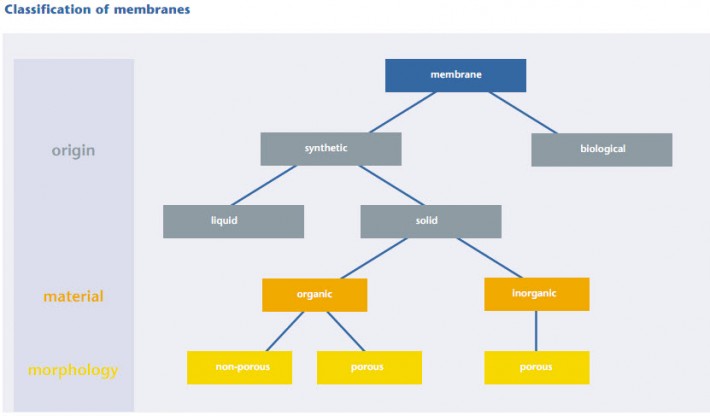
Material separation by means of membrane technology is a physical separation process. Compared with other separation technologies, this technology has the advantage that the separated materials are neither thermally nor chemically or biologically modified. The fields of application of membrane processes stretch from simple filtration of solids, e.g. separation of activated sludge in municipal waste water treatment, up to the separation of materials within the molecular range, e.g. retention of dissolved salts in seawater desalination. The operating principle of a membrane can be described in the wider sense like that of a filter. As shown in fingure, a substance mixture, called feed or raw solution (e.g. raw waste water) is separated by the membrane. The part which passes through the membrane almost unhindered is called permeate or filtrate. To waste water purification the permeate represents the treated phase. The portion retained by the membrane is the brine or concentrate. | The driving force for the separation process is the pressure difference between the feed and permeate side, the so-called transmembrane pressure difference or transmembrane pressure. It is applied by overpressure on the side of the feed or low pressure on the side of the permeate. Dependent on the membrane employed, the transmembrane pressure is between 0.1 bar and 70 bar, in special cases it is up to 120 bar. The characteristics selectivity and capacity are of decisive importance for the economic efficiency of a membrane process. The selectivity describes the ability of a membrane to differentiate between the components of a mixture and thus to separate one phase from the other. By capacity of a membrane, we understand the flow under specific operational conditions. The flow is defined as the volumertric flow rate per unit surface area (unit: l/(m2.h)) | ||||||
 | |||||||
| Another important feature of a membrane is described by the parameter permeability. It is defined as the quotient from flow and the accompanying transmembrane pressure (unit: l/m2.h.bar)). The permeability of a membrane is influenced by the membrane condition and the filtration characteristics of the waste water. The latter depend on the material composition and the characteristics of the waste water mixture, e.g. temperature, particle-size distribution and viscosity. | |||||||
| Membrane processes in Waste Water purification | |||||||
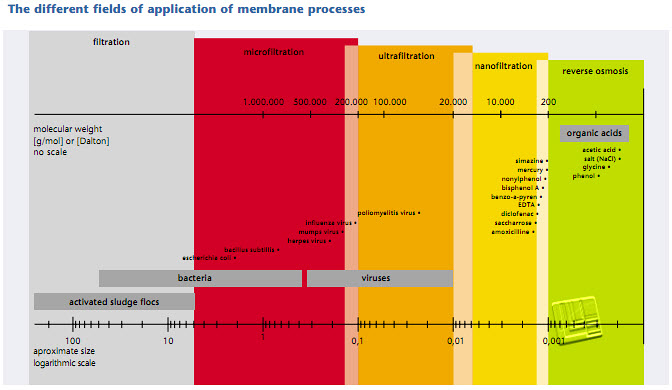
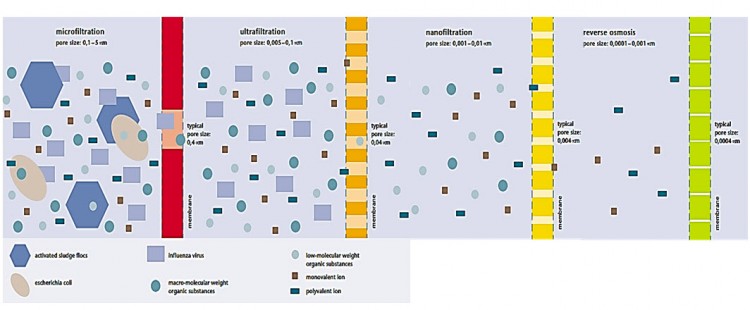
There are various membrane processes which differ in their molecular separation size and the driving force which has to be expended. Which process is employed depends on the waste water composition and the separation goal. The separation goal in municipal waste water purification is above all the separation of the cleaned waste water from the biomass in order to meet the effluent standards. In an industrial company, the employment of a membrane process for waste water purification may be feasible, particularly if a useful integration into the production process is possible. Besides the treatment of the waste water, it is also frequently aimed at reusing the permeate and possibly the concentrate, so that these can be recycled into the production process. In municipal waste water purification, the membrane processes microfiltration (MF) and ultrafiltration (UF) are used. For industrial waste water purification, nanofiltration (NF) and reverse osmosis (RO) are also of importance. These four processes are therefore described in the following. Figure indicates the molecular weight and the size of the meterial which can be separated by microtration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO). The size of some waste water constituents and the pore size of the membranes applied are presented in Figure.
| |||||||
Table provides an overview of the membrane processes presented, with driving force and application possibilities. Further details about the individual processes are given in the following sections.
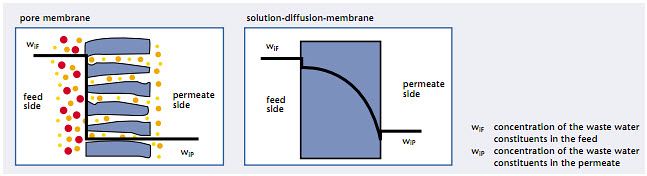
Separation by pore membranes (MF, UF) is based on a sieving effect, while differences in solubility and diffusivity are responsible for the selectivity of solution-diffusion membranes (NF, RO) | The concentration process of a component to be separated by a membrane is represented in an idealized manner for a pore membrane and a solution-diffusion membrane in Figure. With the pore membrane, the component to be separated is retained by the membrane due only to its size. In the course of concentration, a sharp separation on the membrane surface can be recognized. When entering into the membrane, the concentration of the component in the feed drops down to the concentration in the permeate. However, with a solution-diffusion membrane a reduction of the concentration also takes place within the membrane due to the transportation mechanisms.
| ||||||
| |||||||
| |||||||
 | |||||||